Natural Gas Freezing Point Ppt And Boiling Graph Aka Phase Change Diagram Or
Luckily, even though gas freezing poses a potentially serious problem, the freeze point of natural gas is extremely low. So, if you have natural gas heating or a stove, you shouldn’t Here is all you need to know about how, when, and why natural gas freeze can occur.
What Are the Freezing, Melting, and Boiling Points of Solids, Liquids
Natural gas itself does not freeze under typical atmospheric conditions. Which makes it almost impossible to freeze this gas. This means that the gas remains in its gaseous state even in the harshest winter weather.
At this point though, it’s always important to remember that natural gas isn’t what’s freezing.
The freezing point of natural gas is not a straightforward answer as it is a mixture of many different elements. Natural gas cold weather safety can natural gas pipes freeze? The chances are slim that the natural gas flowing through the pipes leading to the meter at your home or business will freeze. In its unrefined state, natural gas does often contain water molecules and other chemicals that can freeze—this was an issue in 2021 in texas where the
The natural phenomenon of freezing is a common occurrence in the operation of a natural gas pipeline system. Whether the gas is “produced gas” from a crude oil well, or “natural gas” from a gas well, the possibility for hydrates and the resultant problems, is real. Freezing is a potential and serious problem starting at the Think of all the natural gas pipelines in the us, all the wellheads located in cold or warm climates.

There is no discrimination over where you live—a freeze up can happen anywhere, at anytime.
The natural phenomenon of freezing is a common occurrence in the operation of a natural gas pipeline system. Freezing is a potential and serious problem starting at the production wellhead through the last point in the customer delivery system. Natural gas is a mixture of gases, primarily methane. Methane, like propane, doesn't freeze in the way water does.
It transitions from a gas to a liquid and then to a solid at extremely low temperatures. Point q 2 is the upper quadruple point, where four phases (liquid water, liquid hydrocarbon, gaseous hydrocarbon, and solid hydrate) are found in equilibrium. Point q 1, the lower quadruple point, typically occurs at 32 °f (ice freezing point) where four phases (ice, hydrate, liquid water, and hydrocarbon gas) are found in equilibrium. While carbon dioxide easily solidifies at cryogenic conditions, it also easily transforms into vapor.

For instance, the triple point temperatures of r134a and r290 (propane) are 169.85 k and 85.53 k, respectively.
Dry ice and water ice form a virtually pure co2 and water solid phase, respectively. The natural phenomenon of freezing is a common occurrence in the operation of a natural gas pipeline system. Whether the gas is “produced gas” from a crude oil well, or “natural gas” from a gas well, the possibility for hydrates and the resultant problems, is real. Freezing is a potential and serious problem starting at the
So, freezing gas is probably not a worry, even in the uk’s worst winters at home! Product and company identification company phone number: Liquefied natural gas material use: While a gas flow stream may operate normally with an internal temperature above freezing and an external temperature below freezing, the internal temperature could drop below freezing with a reduction in pressure.
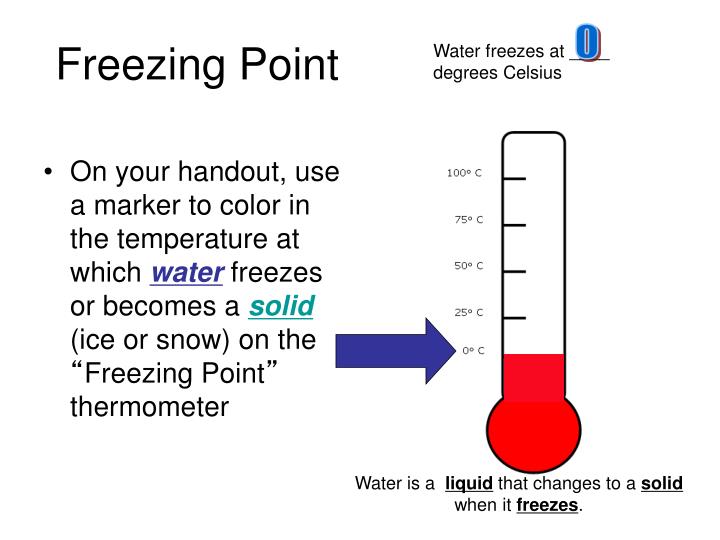
If the gas has any water vapor or condensate present, freezing can occur.
Natural gas production in texas fell from around 21 billion cubic feet of gas to less than 14 billion, and gas moving from more than two dozen west texas processing plants to pipelines fell by 85% In some cases, specific additives can raise the freezing point. This is less common but can occur when the additive enhances the formation of a solid structure. Freezing point depression and elevation in mixtures.
The freezing point of a gas can be significantly altered when it is part of a What causes water in natural gas lines? So, it is highly unlikely that your gas lines will be exposed to this temperature and freeze. Whether from gas transfer, weather, or temperature change, moisture can be damaging to a gas line.
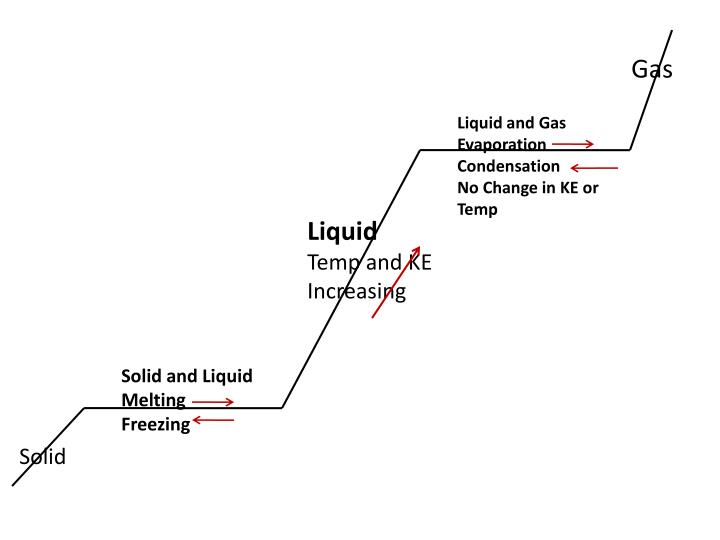
Freezing weather threatens the integrity of gas pipelines every year.
While this once seemed like a problem reserved for northern areas, severe weather events like the historic cold snap that gripped the central and southern u.s. For more than a week in february show that even pipes in subtropical climates aren’t protected from freezing weather and its effects. Methane is a gas at standard conditions. However, at low temperature and/or high pressures the gas becomes a liquid or a solid.
The methane phase diagram shows the phase behavior with changes in temperature and pressure. The curve between the critical point and the triple point shows the methane boiling point with changes in pressure. But what is the natural gas freeze point? Every fuel or gas has a freezing point, right?